Sleep problems are frequently seen in several chronic pain populations (Pachman et al. 2012;Raizenblatt et al. 2011; Buchwald et al. 1994; Parmelee et al. 2015; Sezgin et al. 2015; Purushothaman et al. 2013; Tang et al. 2007). While the function of sleep is not yet fully discovered, it is known that a lack of sleep can have a negative impact on the general health. Common consequences of sleep deprivation are concentration and memory issues, obesity, cardiovascular diseases, high blood pressure, depression, pro-inflammatory state and metabolic disorders (Chua et al. 2017; Gumenyuk et al. 2014; van Heugten-van der Kloet et al. 2014; Alberca-Reina et al. 2015; Miller et al. 2007; Alvarez et al. 2004; Balkin et al. 2008). Ample evidence indicates a bidirectional relationship between pain and sleep (McBeth et al. 2015;Finan et al. 2013). However, sleep problems are poorly assessed and targeted in current treatment of chronic pain patients (Cheatle et al. 2016; Tang et al. 2007). It is suggested that interventions targeting sleep in patients with chronic pain may produce improvements in pain symptoms (Cheatle et al. 2016; Tang et al. 2007; Finan et al. 2014). To improve sleep, a good understanding of sleep regulation mechanisms and influencing factors is needed (Cheatle et al. 2016;Nijs et al. 2018).
Sleep is mainly controlled by two processes: 1) Sleep homeostasis or sleep pressure (Process S)and the inner/body clock or the circadian clock (Process C)(Borbély et al. 2016;Goel et al. 2013; Dierickx et al. 2016; Fisher et al. 2013).
The homeostatic process (process S)is driven by sleep debt. While awake, the sleep debt increases until the sleep pressure is high enough to fall asleep. Once asleep, the sleep debt declines until awakening is triggered due to both low sleep pressure (process S) and increasing alertness (process C; see following paragraph). Napping throughout the day leads to lower sleep pressure at the end of the day and possible sleep difficulties. On the other hand, restricting sleep and avoiding taking naps will increase sleep debt, resulting in higher sleep pressure (Borbély et al. 2016;Goel et al. 2013; Dierickx et al. 2016; Fisher et al. 2013).
The circadian clock (Process C)is an internal timekeeping system with a near-24h period. It is an underlying mechanism regulating different processes of the body at a molecular level (Borbély et al. 2016;Dierickx et al. 2016; Fisher et al. 2013; Dierickx et al. 2018; Wever et al. 1975; Wright et al. 2001). Almost every tissue of our body has its own peripheral clock, sometimes even at single-cell level. These clocks help to maintain local circadian tissue physiology (Dierickx et al. 2016; Fisher et al. 2013; Dierickx et al. 2018). The central core clock, also called “Master clock”, is located in the suprachiasmatic nucleus (SCN), a part of the anterior hypothalamus in the brain, and synchronises all peripheral clocks (Borbély et al. 2016; Dierickx et al. 2016; Fisher et al. 2013; Dierickx et al. 2018).
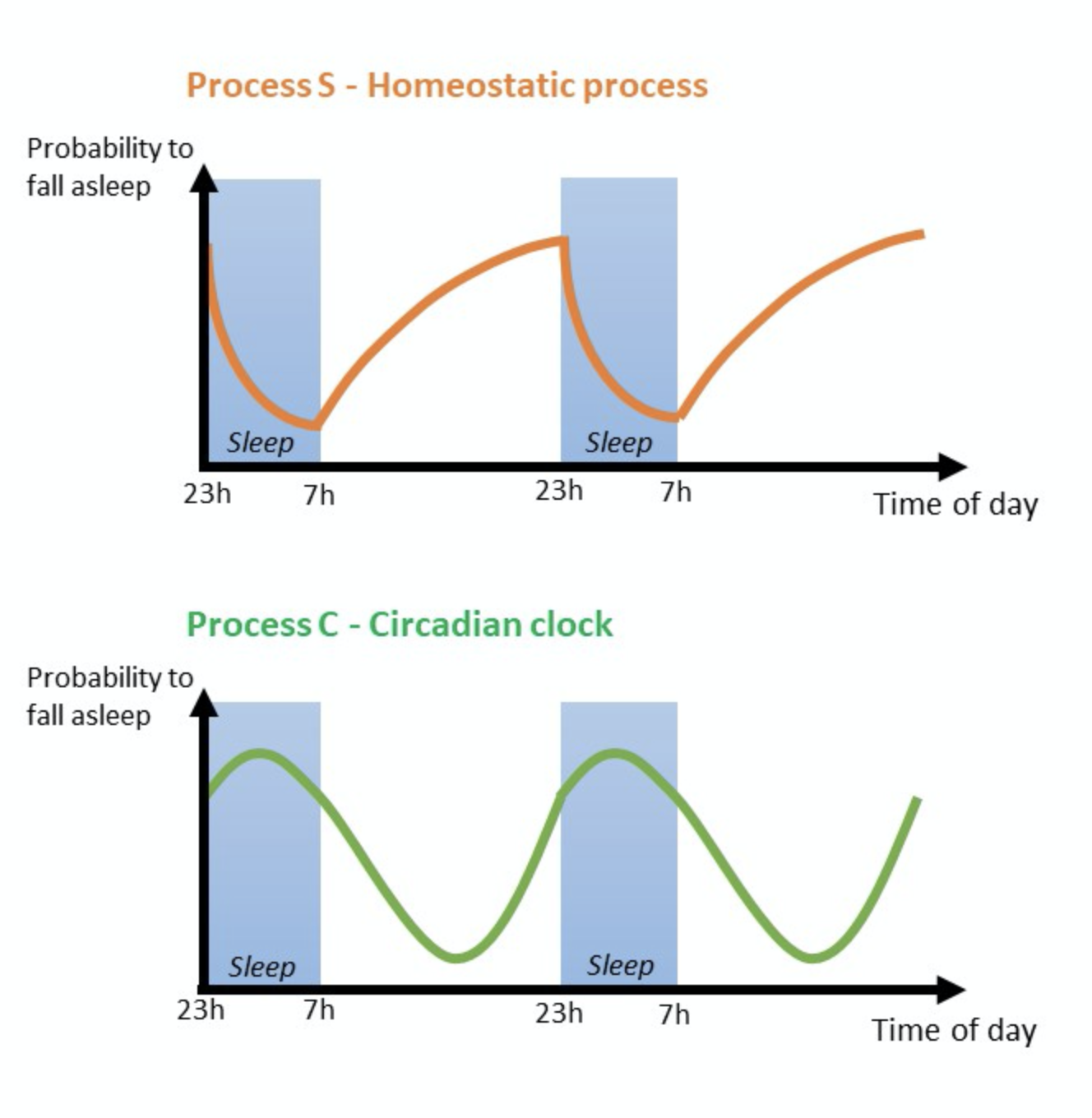
This whole process (Process C) makes us feel less alert at night and more alert at day (Goel et al. 2013;Fisher et al. 2013). This also explains why some people (e.g. shift workers) have difficulties to fall asleep in the morning after they stayed awake for a whole night.
Our daily life consists of a 24-h cycle of day and night. Although, when there would be no external cues (e.g. temperature, light, food metabolites, …), the cycle of most individuals would generally be one hour longer (Wever et al. 1975; Wright et al. 2001). The circadian clock relies on those external cues, also known as “Zeitgebers”, for synchronisation to our surroundings.
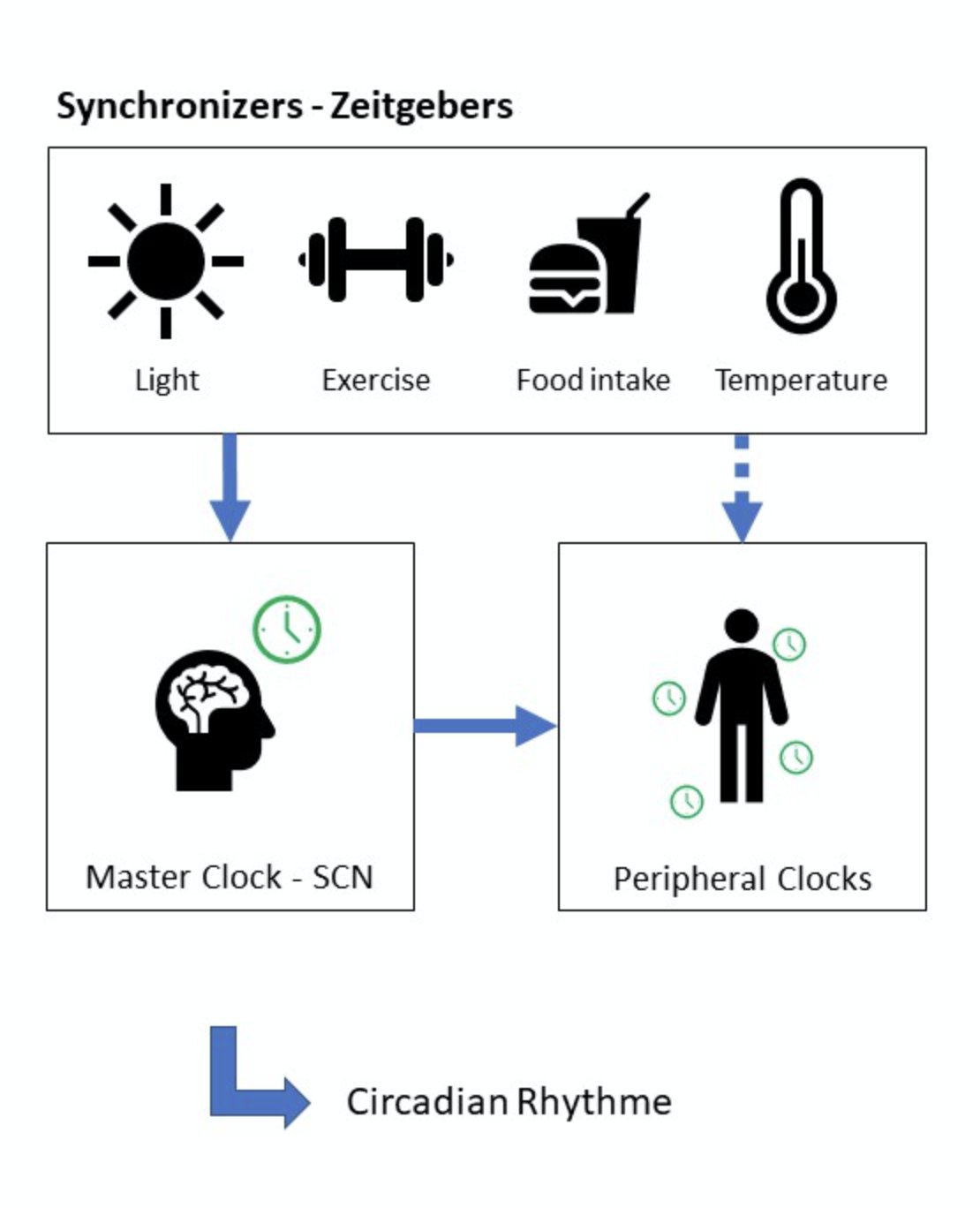
Light is the main synchronizer, forcing us to adapt to a 24-h period. It is possible to advance or delay our circadian rhythm by adapting the light exposure and other synchronizers (Dierickx et al. 2016; Wever et al. 1975; Wright et al. 2001; Wright et al. 2013). When you expose yourself to light in the morning (e.g. morning walk after sunrise), you are more likely to advance your rhythm. This could be useful to reduce or prevent jetlag (Eastman et al. 2005). However, when you expose yourself to light late in the evening, you’re most likely delaying your rhythm (Wever et al. 1975;Wright et al. 2001; Wright et al. 2013). This is what happens during the weekend with many people, particularly late chronotypes. They stay up later than usual and delay their clock, resulting in a “social jetlag” the following Monday (Wittman et al. 2006). Besides, the adaption to cues from the environment is limited to certain level. When the rhythm of the environmental cues changes to fast, the circadian clock does not have the time to adapt and a misalignment will occur (Eastman et al. 2005).
It is important for clinicians and patients to realize that the things we do during the day impacts our sleep at night and vice versa. Most patients will complain about their sleep when they do not feel rested and awake during the day. These patients mostly show inadequate sleep habits and behaviour to compensate their bad sleep (e.g. napping in the afternoon). By giving insight in the mechanisms of sleep regulation, the first steps are made to address and adapt inadequate sleep behaviour (Cheatle et al. 2016;Nijs et al. 2018).
Thomas Bilterys
Thomas Bilterys is a doctoral researcher at the Vrije Universiteit Brussel (Brussels, Belgium) and Ghent university (Ghent, Belgium). His research and clinical interest goes out to chronic pain and associated sleep problems.
2018 Pain in Motion
References and further reading:
https://www.ncbi.nlm.nih.gov/pubmed/23008320
https://www.ncbi.nlm.nih.gov/pubmed/21594765
https://www.ncbi.nlm.nih.gov/pubmed/8148456
https://www.ncbi.nlm.nih.gov/pubmed/25283955
https://www.ncbi.nlm.nih.gov/pubmed/25322735
https://www.ncbi.nlm.nih.gov/pubmed/23629992
https://www.ncbi.nlm.nih.gov/pubmed/17309767
https://www.ncbi.nlm.nih.gov/pubmed/29166387
https://www.ncbi.nlm.nih.gov/pubmed/24587577
https://www.ncbi.nlm.nih.gov/pubmed/25462597
https://www.ncbi.nlm.nih.gov/pubmed/26302671
https://www.ncbi.nlm.nih.gov/pubmed/17430213
https://www.ncbi.nlm.nih.gov/pubmed/18779203
https://www.ncbi.nlm.nih.gov/pubmed/25604572
https://www.ncbi.nlm.nih.gov/pubmed/24290442
https://www.ncbi.nlm.nih.gov/pubmed/27208716
https://www.ncbi.nlm.nih.gov/pubmed/17309767
https://www.ncbi.nlm.nih.gov/pubmed/25477769
https://www.ncbi.nlm.nih.gov/pubmed/29425327
https://www.ncbi.nlm.nih.gov/pubmed/26762182
https://www.ncbi.nlm.nih.gov/pubmed/23899598
https://link.springer.com/chapter/10.1007/978-3-319-25427-2_5#citeas
https://www.ncbi.nlm.nih.gov/pubmed/23604479
https://www.ncbi.nlm.nih.gov/pubmed/29258993
https://www.ncbi.nlm.nih.gov/pubmed/1193771
https://www.ncbi.nlm.nih.gov/pubmed/11717461
https://www.ncbi.nlm.nih.gov/pubmed/23910656
https://www.ncbi.nlm.nih.gov/pubmed/15700719
https://www.ncbi.nlm.nih.gov/pubmed/16687322
Free PDF available from:
Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013 Dec;14(12):1539-52.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4046588/pdf/nihms521705.pdf
Finan PH, Buenaver LF, Coryell VT, Smith MT. Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med Clin. 2014 Jun 1;9(2):261-274.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4248667/pdf/nihms591095.pdf
Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and Managing Sleep Disturbance in Patients with Chronic Pain. Anesthesiol Clin. 2016 Jun;34(2):379-93.
https://www.anesthesiology.theclinics.com/article/S1932-2275(16)00008-2/pdf
Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155-90.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3963479/pdf/nihms562935.pdf
Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016 Apr;25(2):131-43.